Melanoma Genomics
October 30, 2012Mohammed Kashani-Sabet
[M. Kashani-Sabet, "Melanoma Genomics," in Targeted Therapeutics in Melanoma, T. F. Gajewski and F. S. Hodi, Eds., ed New York: Springer, 2012, pp. 17-25.]
- Melanoma is an ideal clinical model to apply genomic analysis to study Tumor Progression.
- Melanoma Progression has readily defined clinical phases with Histological Correlations.
- Clarks et al. [1] described successive stages of Melanoma Progression Classic Model.
- benign melanocytic proliferation yields a nevus.
- which can transform into primary melanoma, beginning in the radial growth phase.
- with a capacity to progress to the vertical growth phase, and culminating in metastatic
melanoma. - the vertical growth phase has been postulated to be required for metastatic competency.
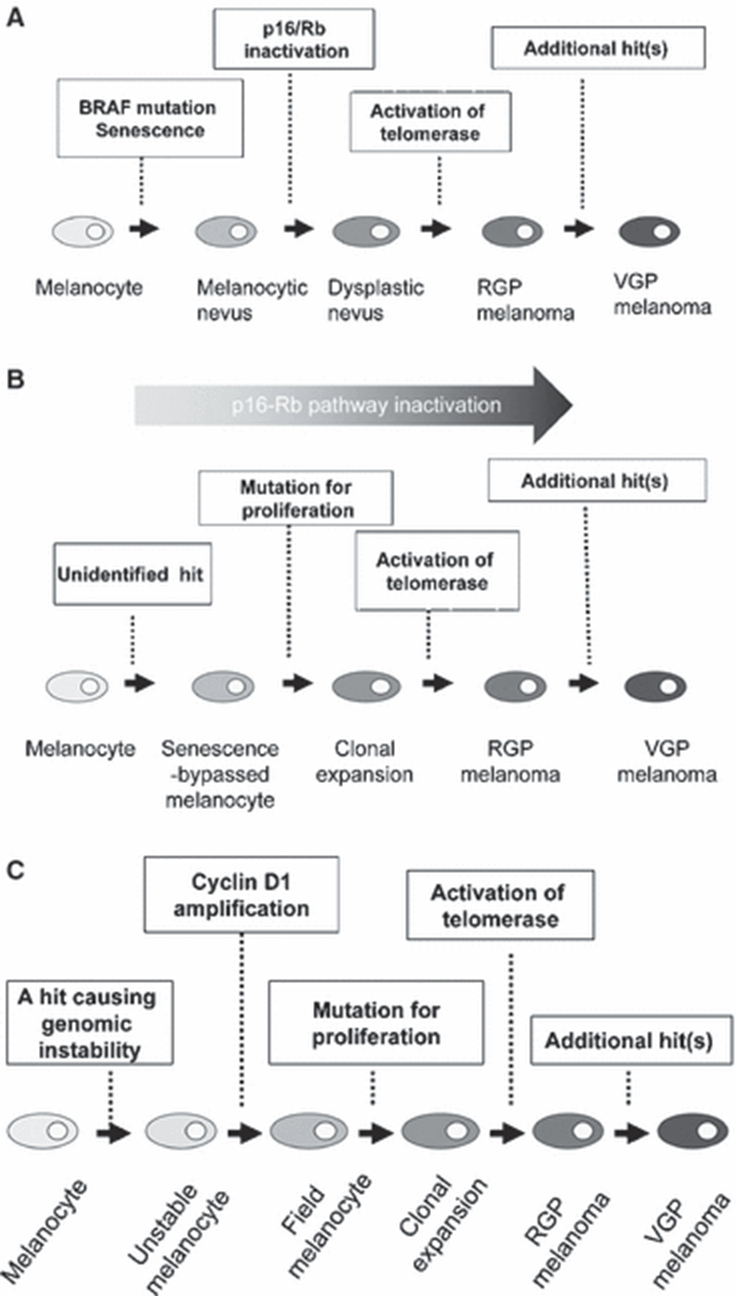
Figure 1.
(A) A genetic model of melanoma development and progression based on the Clark model (adopted from Bennett, 2003; and Miller and Mihm, 2006).
This model emphasizes a series of histopathological changes beginning
from benign melanocytic nevus to melanoma via dysplastic nevus, and
predicts that BRAF mutation is a crucial step of initiation of
melanocytic neoplasia. Progression from benign melanocytic nevus to
dysplastic nevus requires escape from senescence mediated through p16INK4a-Rb pathway inactivation.
(B) A genetic model of de novo melanoma development (adopted from Michaloglou et al. (2008) with modifications). The first event would be an unidentified hit, which might collaborate with inactivation of p16INK4a-Rb pathway. When a melanocyte already suffering from this hit acquires a proliferative mutation, such as BRAFV600E,
it might fail to undergo oncogene-induced senescence and clonally
proliferate.
(C) A hypothetical genetic progression model of acral melanoma. Normal melanocytes, which acquired genomic instability and CCND1 amplification give rise to field cells, the precursors of RGP melanoma. Progression from field cells to RGP melanoma may require mutations for proliferation, such as KIT, and immortalization. RGP, radial growth phase; VGP, vertical growth phase.
Pigment Cell & Melanoma
Research
Volume
23, Issue 1, pages
64-71, 25 SEP 2009 DOI: 10.1111/j.1755-148X.2009.00645.x
http://onlinelibrary.wiley.com/doi/10.1111/j.1755-148X.2009.00645.x/full#f1
[1] Clark WH, Elder DE, Guerry IV D, et al. A study of tumor progression: the precursor lesions of superfi cial spreading and nodular melanoma. Hum Pathol. 1984;15:1147–65.
Posted by Mohammad Nazmul Haque. Posted In : Research